KEY FEATURES OF OUR ANTIGEN TESTS
Access Bio, Inc.
The CareStart™ COVID-19 Antigen Test
Made in the USA
Provides positive/negative results in 10-15 minutes in non-lab environments
Useful aid in the diagnosis of active COVID-19 infection
Ideal high volume screening device to compliment limited and higher cost RT-PCR tests
Indication for Use: Lateral flow immunoassay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2
Shelf Life: 6-12 months
No instrument required
Can be performed using human nasal swab specimen within the first 5 days of COVID-19 symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
Authorized Settings: CLIA Waived at the Point of Care (POC); High Complexity Labs, Moderate Complexity Labs
Abbott Diagnostics Scarborough, Inc.
The BinaxNOW™ COVID-19 Ag Card Antigen Test
Made in the USA
Provides positive/negative results in 15 minutes in non-lab environments
Useful aid in the diagnosis of active COVID-19 infection
Ideal high volume screening device to compliment limited and higher cost RT-PCR tests
Indication for Use: Lateral flow immunoassay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2
Shelf Life: 6-12 months
No instrument required
Can be performed using human anterior nasal swab specimen within the first 7 days of COVID-19 symptom onset.
Authorized Settings: CLIA Waived at the Point of Care (POC); High Complexity Labs, Moderate Complexity Labs
BENEFITS
Access Bio, Inc.
The CareStart™ COVID-19 Antigen Test
TEST ANYTIME & ANYWHERE
No need for laboratory environment
Visual read results in 10 minutes
No special equipment required
Pre-calibrated buffer bottle
#1 Positive quality control swab (non-infectious) per pack of 20
#1 Negative quality control swab per pack of 20
EASY SAMPLE COLLECTION
Fast and non-invasive
Nasal specimen collection by healthcare professional
HIGH ACCURACY*
Sensitivity: 87.2% (AN) / 93.8% (NP)**
Specificity: 100% (AN) / 99.3% (NP)**
Accuracy: 94.6% (AN) / 98.3% (NP)**
LOW COST
Room temperature storage
Long life high stability immunoassay
Light weight shipping
Abbott Diagnostics Scarborough, Inc.
The BinaxNOW™ COVID-19 Ag Card Antigen Test
TEST ANYTIME & ANYWHERE
No need for laboratory environment
Visual read results in 15 minutes
No special equipment required
#1 Positive quality control swab (non-infectious) per pack of 40
EASY SAMPLE COLLECTION
Fast and non-invasive
Anterior nares specimen collection by healthcare professional
HIGH ACCURACY*
LOW COST
Room temperature storage
Long life high stability immunoassay
Light weight shipping
**AN=Anterior Nares; NP=Nasopharyngeal
SUPPORT DOCUMENTS
Access Bio, Inc.
The CareStart™ COVID-19 Antigen Test
Abbott Diagnostics Scarborough, Inc.
The BinaxNOW™ COVID-19 Ag Card Antigen Test
GENERAL COVID-19 DOCUMENTS
PRICING
Access Bio, Inc.
The CareStart™ COVID-19 Antigen Test
$320 - PACK OF 20
#20 Rapid Immunoassay Tests
#20 Patient Nasal Swabs
#20 Pre-calibrated Extraction Buffer Vials
#1 Positive Quality Control Swab (Non-Infectious)
#1 Negative Quality Control Swab
Intended to be used with nasal swab specimens.
This EUA granted product is available for non-prescription home use.
Abbott Diagnostics Scarborough, Inc.
The BinaxNOW™ COVID-19 Ag Card Antigen Test
$400 - Pack of 40
#40 BinaxNOW COVID-19 Ag Cards
#40 Patient Nasal Swabs
#1 Reagent Bottle (7.5mL)
#1 Positive Quality Control Swab (Non-Infectious)
#1 Negative Quality Control Swab
#1 Product Insert
Intended to be used with nasal swab specimens.
This EUA granted product is available to high complexity CLIA labs, moderate complexity CLIA labs and CLIA waived Point of Care (POC) settings.
FREQUENTLY ASKED QUESTIONS
1. What is the COVID-19 Antigen Test?
Antigen tests detect proteins specific to a virus that appears in infected individuals, which indicates active infection.
2. What are the benefits of using the COVID-19 Antigen Test?
Urgency: Provide presumptive qualitative results onsite in a matter of minutes. Providing healthcare workers with more information at critical moments in the patients care.
Volume: Fast turn-around testing of symptomatic patients can reduce the burden on hospitals and clinics.
Triage Efficiency: Presumptive antigen results for symptomatic patients allow hospitals to reduce doctor/patient interview time, further diminishing the chance of contagion in a hospital setting.
Cost: Effective low-cost alternative to RT-PCR testing
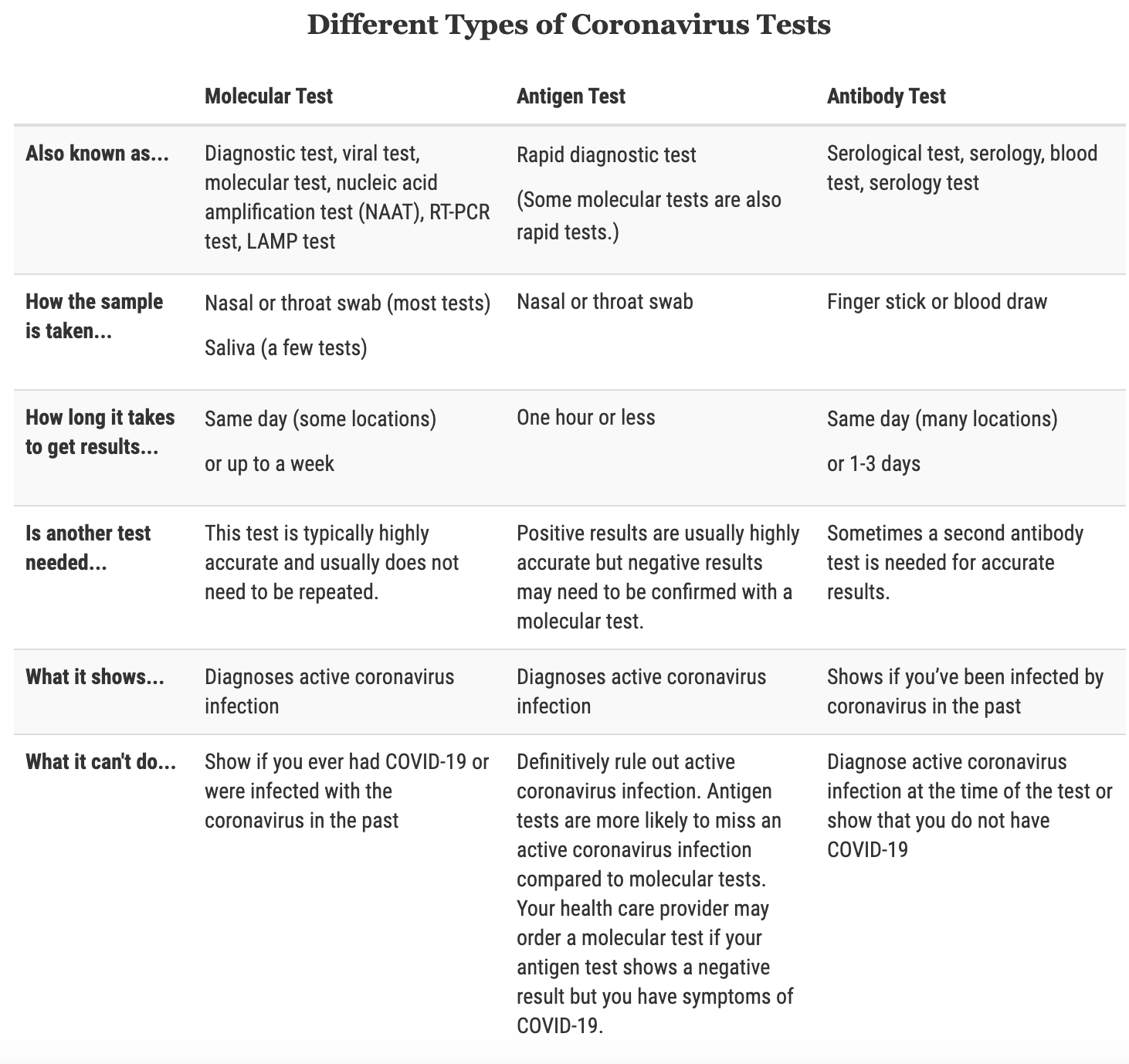
3. What other types of coronavirus tests exist?
4. How do our Antigen Tests work?
Our Antigen Tests detect the presence of viral proteins (antigens) expressed by the COVID-19 virus in a sample from a person’s respiratory tract (Abbott Diagnostics Scarborough/BinaxNOW™: nasal swab; Access Bio/CareStart™: nasal swab). If the target antigen is present in sufficient concentrations in the sample, it will bind to specific antibodies fixed to the test strip enclosed in the cassette and generate a colored line within 10-20 minutes.
The antigen(s) detected are expressed only when the virus is actively replicating; therefore, such tests are best used to identify acute or early infection (within the first seven days of symptom onset).
The test line region of the COVID-19 Antigen Tests are coated with Anti-SARS-CoV-2 antibody. The strip has the follow detection lines:
T - is fixed with the with the anti-SARS-CoV-2 antibody
C - is fixed with the quality control antibody
During testing, the specimen reacts with anti-SARS-CoV-2 antibody-coated particles in the reaction pad. The mixture then migrates upward on the membrane by capillary action and reacts with the anti-SARS-CoV-2 antibody in the test line region (T).
If the specimen contains SARS-CoV-2 antigens, a colored line will appear in test line region (T) as a result of antigen capture. If the specimen does not contain antigens to SARS-CoV-2, no colored line will appear in the test line region (T), indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region (C), indicating that the proper volume of sample has been added and membrane wicking has occurred.
5. What is the significance of our COVID-19 Antigen Test results?
Presumptive Positive (Line appears in T line area) = Patient has active SARS-CoV-2 infection. [1]
Presumptive Negative (No line in T line area) = Patient does not have active SARS-CoV-2 infection. [2]