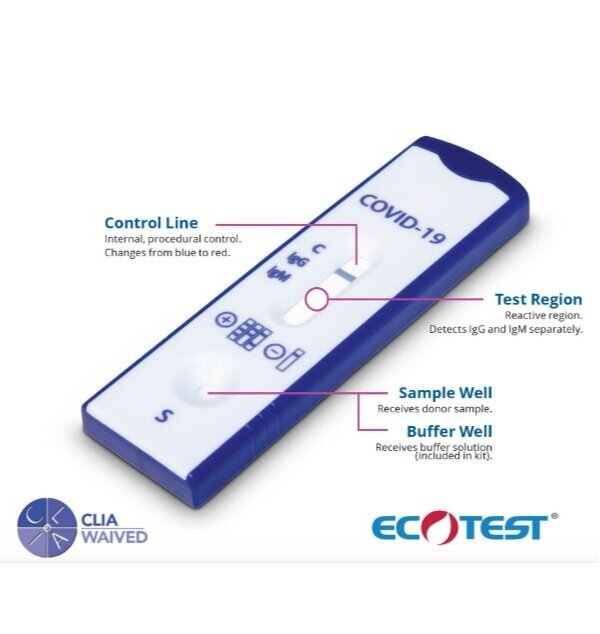
KEY FEATURES OF OUR ANTIBODY TESTS
HEALGEN TEST
Provides positive/negative results in 10-15 minutes.
Increased screening with IgM and IgG antibody detection.
Ideal high volume screening device to compliment nucleic acid tests.
Indication for Use: Lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2.
Clinical Sensitivity: 93.87%
Clinical Specificity: 99.10%
Relative Accuracy: 97.19%
# Tests vs RT-PCR (n): 704
Shelf Life: 24 months
No instrument required
Made in China
CLIA Authorized Settings: High Complexity Labs, Moderate Complexity Labs
Can be performed using either venous whole blood, serum or plasma (not finger stick specimens).
ASSURE TEST
Provides positive/negative results in 10-15 minutes.
Increased screening with IgM and IgG antibody detection.
Ideal high volume screening device to compliment nucleic acid tests.
Indication for Use: Lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2.
Clinical Sensitivity: 100.00%
Clinical Specificity: 98.80%
Relative Accuracy: 99.10%
# Tests vs RT-PCR (n): 110
Shelf Life: 9 months
No instrument required
Made in China
CLIA Authorized Settings: CLIA waived for use with finger stick whole blood specimens at the Point of Care (POC); High Complexity Labs, Moderate Complexity Labs
Can be performed using either venous whole blood, serum, plasma or finger stick specimens.
SUPPORT DOCUMENTS
HEALGEN TEST
ASSURE TEST
GENERAL COVID-19 DOCUMENTS
PRICING
HEALGEN TEST
$200
Pack of 25
includes Test Cassettes, Pipettes and Buffer Solution
Lancets are not included/needed as this test is intended to be used with venous whole blood, plasma, or serum (not finger stick).
This product is only available to high and/or moderate complexity CLIA labs. The purchaser will be asked to provide the CLIA lab license/certificate number at checkout.
ASSURE TEST
$240
Pack of 20
includes Test Cassettes, Pipettes, Lancets, Alcohol Swabs and Buffer Solution
Intended to be used with venous whole blood, plasma, serum or finger stick.
This product is available to CLIA waived point of care (POC) settings.
FREQUENTLY ASKED QUESTIONS
1. What is the COVID-19 IgM-IgG Antibody Test?
Covid-19 IgM-IgG Rapid Test is a lateral flow immunoassay used to qualitatively detect IgG and IgM antibodies to SARS-COV-2 virus in human whole blood, serum or plasma.
2. How does the COVID-19 IgM/IgG Antibody Test work?
The test strip uses a colloidal gold recombinant novel coronavirus antigen and a quality control antibody colloidal gold marker. The strip has the follow detection lines:
IgM - is fixed with monoclonal anti-human IgM antibody for detecting the novel coronavirus IgM antibody.
IgG - is fixeda with monoclonal antihuman IgG antibody for detecting the novel coronavirus IgG antibody.
C - The quality control antibody is fixed on the C (Control) line.
3. How quickly can the COVID-19 IgM/IgG Antibody Test yield results?
Results can be interpreted at 10-15 minutes after sample and buffer are combined in the cassette sample wells.
4. Is the COVID-19 IgM/IgG Antibody Test available for sale in the United States?
Due to the Coronavirus Public Health Emergency, the FDA updated its Policy for Diagnostic Tests for COVID-19 on March 16, 2020. Included in this update is guidance for commercial manufacturers, such as our test, for screening tests that identify antibodies (e.g., IgM, IgG) to SARS-CoV-2 from clinical specimens. On May 4, 2020 and May 11, 2020 it further refined its guidance to read as follows: “Unless and until an EUA is issued that authorizes additional testing environments for a specific test, under CLIA, use of that test is limited to laboratories certified to perform high complexity testing, and at the point-of-care when covered by the laboratory’s CLIA certificate for high-complexity testing. This policy does not apply to at-home testing, including at-home specimen collection, due to additional considerations that require FDA review.” While an application is under review, please note the following information:
• The test has not been reviewed by the FDA.
• Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
• Negative results do not rule out SARS-CoV-2 infection, especially in those who have been exposed to the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in those individuals.
The results from rapid antibody testing should not be used as the sole basis to diagnose or exclude a COVID-19 infection or to determine an individual’s infection status.
5. How accurate are our COVID-19 IgM/IgG Antibody Tests?
Antibody Test (Healgen): The clinical sensitivity is 93.87% and clinical specificity is 99.10% when compared to RT-PCR. The relative accuracy rate is 97.19%.
Antibody Test (Assure): The clinical sensitivity is 100.00% and clinical specificity is 98.80% when compared to RT-PCR. The relative accuracy rate is 99.10%.
6. Can the device be frozen for long-term storage?
The device should never be frozen. If refrigerated, allow the buffer, specimen and device to reach room temperature before use. The advised storage is 2-30⁰C / 36-86⁰F.
7. What is the significance of our COVID-19 IgM/IgG Antibody Test results?
IgM presumptive positive = Indication of onset of acute SARS-CoV-2 infection
IgG/IgM presumptive positive = Indication of active SARS-CoV-2 infection
IgG presumptive positive = Indication of later-stage SARS-CoV-2 infection or possible developed immunity.
Negative = a medical professional should observe symptoms and epidemiology of patients. Retesting should be considered if symptoms appear or persist.
The Covid-19 IgM/IgG Antibody Test can be used to screen patients suspected of having been affected by the novel coronavirus. However, results of this test should not be the only basis for diagnosis. Results should be used in combination with clinical observations and other testing methods such as nucleic acid PCR test.
8. What other types of coronavirus tests exist?
9. Who can buy and use the Covid-19 IgM/IgG Antibody Tests?
Antibody Test (Healgen): Professional Health Care institutions only. The Covid-19 IgM/IgG Rapid Test is NOT for home use. Use of this test is limited to laboratories certified to perform high complexity and moderate complexity testing, and at the point-of-care when covered by the laboratory’s CLIA certificate for high-complexity or moderate-complexity testing.
Antibody Test (Assure): Professional Health Care institutions only. The Covid-19 IgM/IgG Rapid Test is NOT for home use. Use of this test is limited to laboratories certified to perform high complexity and moderate complexity testing, and also authorized for use with fingerstick whole blood specimens at the Point of Care (POC), i.e. in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
10. What are the benefits of using the Rapid Test COVID-19 IgM/IgG Antibody Test?
Urgency – The Covid-19 IgM/IgG Antibody Test can provide presumptive qualitative results onsite in a matter of minutes. Providing healthcare workers with more information at critical moments in the patients care.
Volume – Low-cost fast turn-around testing of symptomatic patients can reduce the burden on hospitals and clinics.
Triage Efficiency – Presumptive antibody results for symptomatic patients allow hospitals to reduce doctor/patient interview time, further diminishing the chance of contagion in a hospital setting.
Immunity Screening – Recovering or recovered patients can use the Covid-19 IgM/IgG Antibody Test in conjunction with approved nucleic acid tests to confirm recovery and the existence of virus fighting IgG antibodies in the blood stream.